Embryo collection - Agarose for OPT
Introduction
We have developed a protocol that not only gives improved results in the final 3D reconstruction but also improves the histological serial sections. To aid the reconstruction all embryos that are potential 3D Atlas models are scanned using optical projection tomography (OPT).
We have routinely found that OPT scans of embryos containing residual blood give poor OPT output. This is due to the blood absorbing light resulting in a saturated signal which in turn leads pixel distortion. We have also found that embryos that are fixed in Bouins fixative which are not washed, can once sectioned have a brittle appearance with marked damage to all cells but particularly to the surface ectoderm.
A side by side comparison between 2 embryos, not washed and washed -
| not washed | washed |
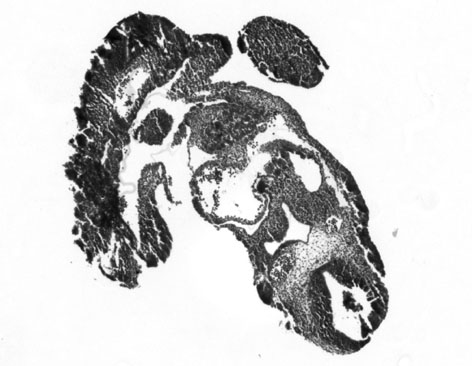 |
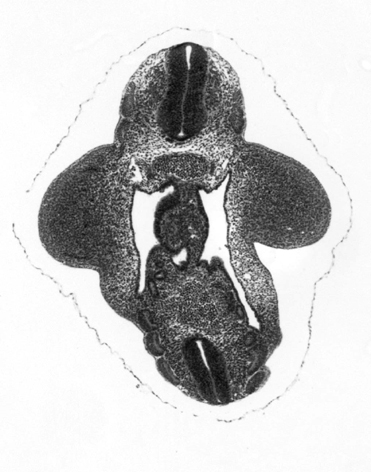 |
We routinely dissect embryos at room temperature in PBS. Dissection at room temperature allows the heart to continue pumping. We have also found that delay in cutting the umbilical cord with dissection scissors also assists in bleeding the embryo. Post fix washing in sheep serum and TBST should remove the rest of the blood. All embryos for the e-Mouse Atlas Project that are selected for processing will be dissected at room temperature in room temperature PBS.
Theiler Stage models Ts15 , Ts16 , Ts17, and Ts18 have had the amnion left intact as we have found that this not only protects the surface ectoderm of the embryo but it is critical in retaining the posture of the embryo as it progresses through the processing steps and finally into wax.
| ts18 amnion intact |
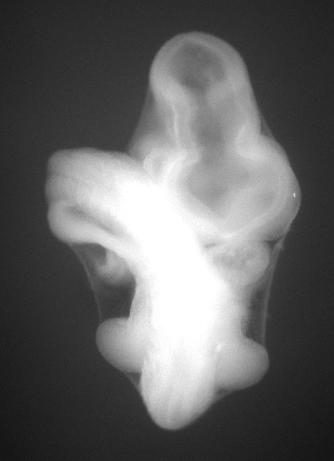 |
The example below shows Ts16 littermates where embryo I has had its amnion removed and post washing, the embryo shows a conformational change. Through the subsequent processing steps and until finally embedded in wax, the embryo will continue to distort making it very difficult to make a 3D reconstruction. Embryo I would be discarded and the selection of the actual embryo is due to a strict criterion examining the quality of OPT data , serial sections , histology and exact Theiler stage.
| ts16 litter mates |
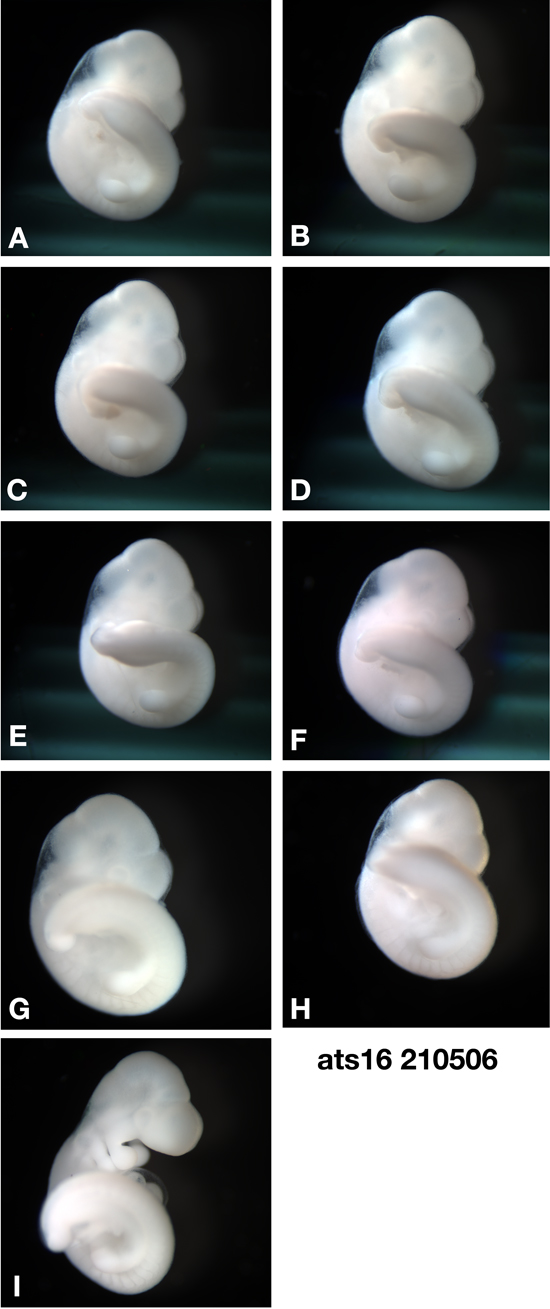 |
Protocol
Embryo collection
The Mouse Atlas Project uses embryos from crosses between F1 hybrid (C57Bl x CBA) mice.
Dissect embryos at room temperature in room temperature PBS.
Theiler stage the embryos (ref) and store in groups of at least 4 identical littermates or more if available.
Fix embryos o/n in Bouins at 4oC
Wash 2hrs 10% SS / 1x TBST / 0.1%NaAz on rotating table at 4oC
Wash with hourly changes 1 x TBST 4oC on rotating table
Continue to wash in 1 x TBST 4oC for at least 5 days/1 week on rotating table
Through 10% 30% 50% 70% ethanol gradient – embryos can be stored in 70% ethanol and photographed at this point.
Prior to OPT scanning
Select the embryos that will require to be scanned.
Move the embryo through a 50% 30% 10% ethanol series.
Rinse in 0.29M sucrose.
Rinse in cooled 1% LMP agarose no warmer that 30oC.
Set in agarose, trim block , stick with glue to metal mount and leave to dehydrate in methanol o/n
Move into BABB o/n
OPT scan
Prepared specimens can now be OPT scanned
Solutions
Bouins Fixative
- Saturated aquaous picric acid 75ml
- 40% formaldehyde 25ml
- glacial acetic acid 5ml
Sheep Serum
- 100% sheep serum diluted to 10% with 1 x TBST
For 100mls of 10 x TBST
- 8g NaCl
- 0.2g KCl
- 25ml 1M Tris HCl pH7.5
- 1ml Triton-X 100
10 x TBST dilute to 1x in dH2O
- 1.4M NaCl
- 27mM KCl
- 0.25M Tris HCl pH7.5
- 1% Triton-X 100
BABB
- 1:2 solution of Benzyl Alcohol and Benzyl Benzoate